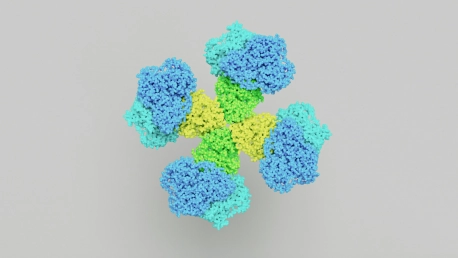
An international team, led by the Max Planck Institute and Philipps University, has made a startling discovery in molecular biology. They’ve found an enzyme in cyanobacteria, known as citrate synthase, which remarkably forms a Sierpiński triangle – a self-repeating fractal pattern. This groundbreaking discovery not only adds an unexpected layer of structural complexity to our understanding of enzymes but also hints at yet-to-be-discovered capabilities of biomolecules. The Sierpiński triangle, which is characterized by nested triangles, poses significant questions about the traditional views of molecular organization, suggesting nature’s molecular design may be more intricate than previously thought. This breakthrough presents a new avenue for exploring the potential of biomolecules, expanding the horizons of molecular biology and biochemistry in unprecedented ways.
Unveiling the Fractal World of Proteins
The research teams were baffled when they first observed the enzyme citrate synthase forming an unanticipated fractal pattern. This phenomenon, known as the Sierpiński triangle, defied the norms of protein geometry, marking the first recorded instance of fractal structures in a naturally occurring protein. It pushed the scientists to rethink the paradigms governing biomolecular organization, as most natural patterns and molecular formations tend to lose their intricacy at larger scales. Yet, here stood a protein maintaining its complexity, irrespective of the magnification, compelling evidence of nature’s ability to embed self-similar patterns within its design.Diving into this microscopic complexity presented significant challenges; the fractal’s recursive geometry confounded the averaging techniques typically employed in electron microscopy. The scientific inquiry ventured into uncharted territory, necessitating the evolution of structural biology methods to accurately decipher and characterize the protein’s enigmatic assembly. As these novel methodologies took shape, they illuminated the elegant choreography of asymmetrical interactions leading to an extraordinary fractal motif—a true deviation from the symmetric interactions that characterize most familiar protein structures.
Asymmetrical Interactions Behind the Fractal Formation
In exploring the intricate landscape of protein structures, scientists have uncovered a remarkable anomaly with citrate synthase proteins: an unconventional asymmetrical interaction. This observation defies the symmetry-centric views of protein organization and embraces a complexity that extends beyond traditional paradigms. These proteins, surprisingly, align in patterns reminiscent of the Sierpiński triangle, displaying fractal-like self-similarity at varying scales. This discovery is groundbreaking as it challenges our fundamental understanding of how proteins assemble and evolve. The existence of such fractal protein structures could revolutionize our comprehension of protein chemistry, calling for a critical reassessment of the principles governing molecular architecture. This exceptional finding suggests that protein complex formation may entail mechanisms far more intricate than previously imagined, potentially opening new frontiers in the study of biological systems.
A Phenomenon Without a Functional Role?
Curiosity then turned towards deciphering the functional significance of this fractal structure within the cyanobacterium’s physiology. The prevailing question was whether this elaborate assembly held any practical advantage. Intriguingly, the study revealed that the fractal formation did not influence the microorganism’s growth, regardless of environmental conditions. This indifference led to the speculative hypothesis that the fractal structure of citrate synthase might be an evolutionary anomaly—mutations accumulated without any selective pressure towards advantage or disadvantage.The findings suggest that such sophisticated structures could emerge in nature, exist undetected, and then vanish, leaving behind no evolutionary trace. Casting a new light on how we perceive molecular evolution, these results potentially point to a world where biological systems harbor a trove of such esoteric configurations, existing simply as a byproduct of the random walk of genetic drift and mutation, rather than a constant drive towards physiological utility.
Tracing the Evolutionary Path of the Fractal Protein
By studying the past, researchers have brought to light the surprising simplicity with which fractal geometries have emerged and vanished within bacterial evolutions, challenging the assumption that such complexities always serve an adaptive role. Their work revolved around reconstructing ancient protein sequences, tracing the lineage of fractals in nature. A particular species caught their attention, suggesting that nature may inherently lean towards creating elaborate forms more often than previously thought.The implications of this study are profound, suggesting that the blueprint of life includes a propensity for intricate, even whimsical structural manifestations. This insight opens up new avenues for scientific inquiry, with the potential to redefine our understanding of biomolecular possibilities. It highlights that the minutiae of life hold within them patterns of staggering complexity, waiting to be discovered. The revelation of the fractal protein beckons the scientific community to look beyond the obvious and probe the depths of nature’s molecular ingenuity.